모두가 양돈 현장에서 PRRS를 여러 차례 겪어 봤지만, 아직도 PRRS 컨트롤에 대한 '정답'은 없습니다. 오랜 기간 양돈농가를 괴롭혀온 만큼 PRRS에 대한 오해와 편견이 많이 쌓여있는 현실입니다. 'PRRS의 모든 지식'(총 15화)을 통해 우리 농장에 맞는 PRRS 컨트롤의 '해답'을 발견할 수 있길 기대합니다. 본 기고글은 HIPRA 본사에서 출간한 'The book for PRRS Knowledge"' 내용을 번역·정리한 것입니다.

1. PRRS 바이러스에 대한 중화항체(NA)가 '비(非)중화항체(중화능력이 없는 항체, non-NA)'보다 약하고 늦게 유도되는 이유는 무엇일까?
이 현상의 원인을 살펴보기 전에 더 중요하게 기억해야 될 부분은 실험실에서 쉽게 ELISA 검사로 확인 가능한 PRRS 항체는 바이러스 방어와 무관하다는 점입니다. 실제 PRRS 바이러스 방어에 관여하는 항체는 이러한 비중화항체의 검출 시기보다 늦게 생성됩니다. 그 원인을 따져보는 것은 농장에 따라 다양한 양상으로 발생하는 PRRS를 컨트롤하는데 유용한 힌트가 될 수 있습니다. 이 현상의 원인은 명확히 규명되진 않았지만, 이론적으로 다양한 가설들이 제시되었습니다. 각 가설들이 제시하는 가능성들은 합당한 근거를 가지고 있으며, 여러 가능성들이 복합적으로 작용하여 일어나는 현상으로 보기도 합니다.
1) 면역세포 표면 분자들의 발현 및 사이토카인 자극을 억제
지난 원고들에서 살펴보았듯 PRRS 바이러스는 항원제시세포(APC)의 기능을 억제하고 Th1 및 Th2 세포의 면역 반응을 방해합니다. 이것이 후천성 면역세포들의 작동 및 이에 따른 중화항체 생성을 지연시킨다는 가설입니다.
2) 면역 반응성이 높은 미끼 항원결정기(decoy epitope)
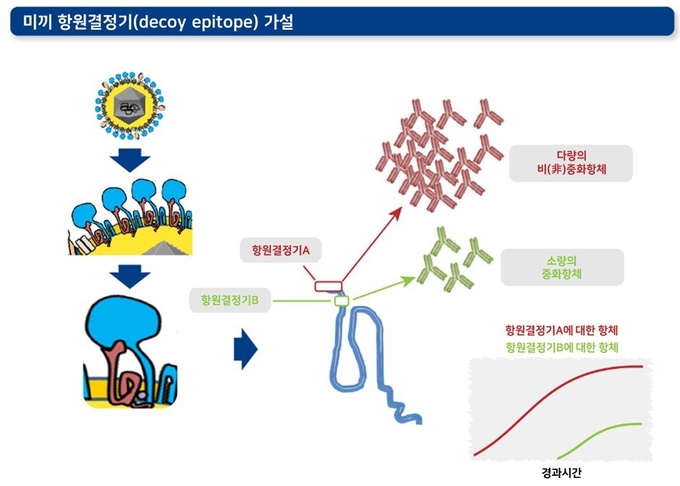
'항원결정기(epitope)'란 면역세포(B세포, T세포)에 인식되는 항원의 특정 부위를 의미합니다. 이 가설에 따르면 바이러스 중화능력이 없는 항체를 많이 형성시키는 '미끼 항원결정기'가 있으며, 이러한 미끼들이 중화항체 형성에 관여하는 주요 항원결정기 근처에 위치하여 방어에 필요한 면역 반응을 억제할 수 있습니다. 이러한 원리에 따라 미끼 항원결정기가 감염 초기에 ‘방어와 관련 없는’ 항체를 형성하는 체액성 면역반응을 강하게 형성하고, 이로 인해 정작 방어에 필요한 중화항체 형성 면역결정기에 대한 반응은 훨씬 뒤로 늦춰집니다.
3) 중화항체 형성 항원결정기의 글리코실화(glycosylation化, 당화)
바이러스의 막단백질에는 보통 '글리칸(glycan)'이라고 하는 당 사슬이 결합되어 있습니다. PRRS 바이러스의 경우 N-글리칸이 결합되어 있습니다. 이 N-글리칸은 중화항체 형성 항원결정기의 내부 혹은 측면에서 방패 역할을 하며 PRRS에 대한 중화항체 형성 및 항체의 특이적 결합을 방해합니다.
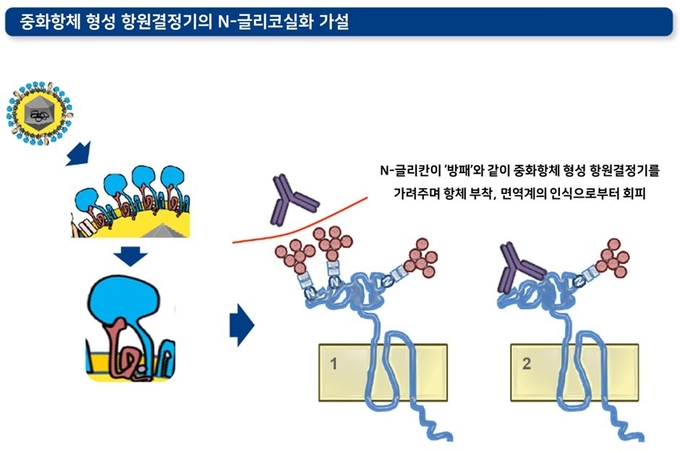
각 PRRS 바이러스에 따라 이러한 글리코실화의 패턴이나 수는 다양합니다. PRRS 바이러스에서 글리코실화가 가능한 부위는 GP3에 6개, GP4에 4개, GP5에 4개(PRRSV1에서 3개, PRRSV2에서 4개)까지 발견되었습니다. PRRS 야외주 바이러스 및 이들의 탈글리코실화 변이주(deglycosylated mutant) 비교 실험은 흥미로운 결과를 보여주며 이 가설을 뒷받침합니다. 탈글리코실화 변이주를 주입한 돼지에서 더 높은 역가의 중화항체가 유도되었을 뿐 아니라, 생성된 중화항체에 대한 감수성 역시 해당 변이주들에서 더 높았습니다.
4) 비정상적인 B세포 증식
PRRS 바이러스는 다중클론성 B세포 활성화(polyclonal B cell activation, 항원과 관계없는 다수의 B세포클론 분열)를 유도하여 림프선이 비대해지는 병증을 일으킵니다(원칙적으로 항원특이적 면역세포는 1종류의 항원수용체를 발현하는 단일 특이성을 가짐). PRRS 바이러스에 감염된 돼지에서는 항원에 감작된 B 세포군(CD2-CD21-)이 두드러지게 감소하며 성숙 및 기억 세포 형성이 억제됩니다.
5) 유전적 다양성
PRRS 바이러스의 유전적 다양성이 매우 높다는 것도 원인일 수 있습니다. 감염이 진행되는 동안 변이주 바이러스의 지속적인 면역 회피가 중화항체의 형성을 방해할 수 있습니다. PRRS 감염 초기에 바이러스의 ORF2-4, ORF5-6 유전자 부위의 다양성에 따라 중화항체에 대한 저항성이 증가하는 것이 확인되기도 했습니다.
2. PRRS 바이러스에 대한 세포매개성 면역반응은 왜 느리고 약하게 일어날까?
해당 현상을 설명하기 위해 역시 다양한 가설들이 제안되었습니다.
1) 특정 사이토카인의 결여 또는 분비 강화
IFN-α 또는 TNF- α와 같은 사이토카인의 분비 억제는 효과적인 Th1 면역 반응 발달에 영향을 미칩니다. 반대로 IL-10의 경우 PRRS 감염에 의해 촉진되며 세포성 면역을 억제하는 작용을 합니다.
2) 적절한 항원 제시 작용 및 T 림프구의 활성 방해
PRRS 바이러스는 항원제시과정 중 면역세포의 표면 분자 발현 맟 T세포의 활성에 관여하는 사이토카인 작용을 방해하여 효과적인 세포성 면역 반응을 억제합니다.
3) T regulatory(Treg) 세포의 작용
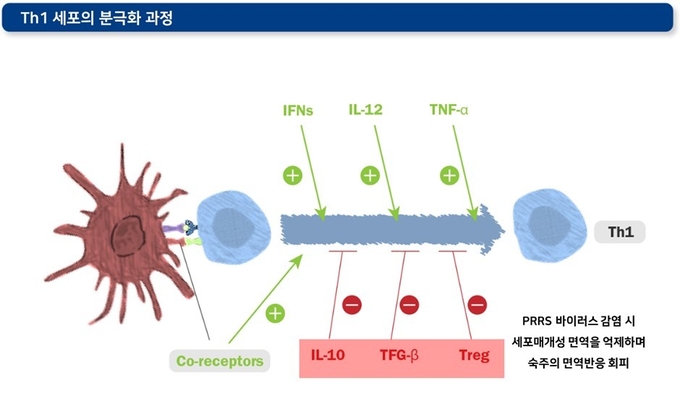
Treg 세포의 주요 기능은 과도한 면역 반응이 일어나지 않도록 예방하는 것입니다. 일부 바이러스들은 이 Treg의 작용을 촉진하거나, IL-10, TGF-β 등과 같은 면역억제성 사이토카인 분비를 촉진하는 방법으로 숙주의 면역 반응을 회피한다는 것이 밝혀졌습니다. PRRSV2에 감염된 경우 Treg 세포들이 TGF-β 생성에 관여하는 림프 조직에서 지속적으로 관찰됩니다. 하지만, Treg 세포와 바이러스 혈증 기간의 관계성은 명확하게 보이지 않습니다. PRRS 바이러스의 N단백질(바이러스 RNA와 작용하여 뉴클레오캡시드를 형성하는 구조단백질)이 Treg의 작용을 조절하는 것으로 보입니다. 한편, PRRS 바이러스가 Treg 세포를 유도하는 능력은 바이러스 분리주에 따라 다르게 나타납니다.
4) 유전적 다양성
중화항체 부분에서 다룬 내용과 유사하게, 변이주의 지속적인 면역 회피 과정은 적절한 세포 매개성 면역의 발달을 방해합니다.

참고 문헌
-
Albina E, Carrat C, Charley B. Interferon-alpha response to swine arterivirus (PoAV), the porcine reproductive and respiratory syndrome virus. J Interferon Cytokine Res. 1998, 18:485-90.
-
Ansari IH, Kwon B, Osorio FA, Pattnaik AK. Influence of N-linked glycosylation of porcine reproductive and respiratory syndrome virus GP5 on virus infectivity, antigenicity, and ability to induce neutralizing antibodies. J Virol. 2006, 80:3994–4004.
-
Badaoui B, Rutigliano T, Anselmo A, Vanhee M, Nauwynck H, Giuffra E, Botti S. RNA-sequence analysis of primary alveolar macrophages after in vitro infection with porcine reproductive and respiratory syndrome virus strains of differing virulence. PLoS One. 2014, 9:e91918.
-
Balasuriya UB, MacLachlan NJ. The immune response to equine arteritis virus: potential lessons for other arteriviruses. Vet Immunol Immunopathol. 2004, 102:107-29.
-
Baumann A, Mateu E, Murtaugh MP, Summerfield A. Impact of genotype 1 and 2 of porcine reproductive and respiratory syndrome viruses on interferon-α responses by plasmacytoid dendritic cells. Vet Res. 2013, 44:33.
-
Bautista EM, Molitor TW. Cell-mediated immunity to porcine reproductive and respiratory syndrome virus in swine. Viral Immunol. 1997, 10: 83-94.
-
Bautista EM, Molitor TW. IFN gamma inhibits porcine reproductive and respiratory syndrome virus replication in macrophages. Arch Virol. 1999, 144:1191-200.
-
Buddaert W, Van Reeth K, Pensaert M. In vivo and in vitro interferon (IFN) studies with the porcine reproductive and respiratory syndrome virus (PRRSV). Adv Exp Med Biol. 1998, 440:461-7.
-
Burgara-Estrella A, Díaz I, Rodríguez-Gómez IM, Essler SE, Hernández J, Mateu E. Predicted peptides from non-structural proteins of porcine reproductive and respiratory syndrome virus are able to induce IFN-γ and IL-10. Viruses. 2013, 5:663-77.
-
Butler JE, Lager KM, Golde W, Faaberg KS, Sinkora M, Loving C, Zhang YI. Porcine reproductive and respiratory syndrome (PRRS): an immune dysregulatory pandemic. Immunol Res. 2014, 59:81-108.
-
Calzada-Nova G, Schnitzlein WM, Husmann RJ, Zuckermann FA. North American porcine reproductive and respiratory syndrome viruses inhibit type I interferon production by plasmacytoid dendritic cells. J Virol. 2011, 85:2703-13.
-
Calzada-Nova G, Schnitzlein W, Husmann R, Zuckermann FA. Characterization of the cytokine and maturation responses of pure populations of porcine plasmacytoid dendritic cells to porcine viruses and toll-like receptor agonists. Vet Immunol Immunopathol. 2010, 135:20-33.
-
Cancel-Tirado SM, Evans RB, Yoon KJ. Monoclonal antibody analysis of porcine reproductive and respiratory syndrome virus epitopes associated with antibody-dependent enhancement and neutralization of virus infection. Vet Immunol Immunopathol. 2004, 102:249-62.
-
Cao J, Grauwet K, Vermeulen B, Devriendt B, Jiang P, Favoreel H, Nauwynck H. Suppression of NK cell-mediated cytotoxicity against PRRSV-infected porcine alveolar macrophages in vitro. Vet Microbiol. 2013, 164:261-9.
-
Chen Z, Zhou X, Lunney JK, Lawson S, Sun Z, Brown E, Christopher-Hennings J, Knudsen D, Nelson E, Fang Y. Immunodominant epitopes in nsp2 of porcine reproductive and respiratory syndrome virus are dispensable for replication, but play an important role in modulation of the host immune response. J Gen Virol. 2010, 91:1047-57.
-
Choi CS, Christianson WT, Collins JE, Joo HS, Molitor T. Antibody dependent enhancement of SIRS virus replication. Am Assoc Swine Pract Newsl. 1992, 4:30.
-
Chung HK, Chae C. Expression of interleukin-10 and interleukin-12 in piglets experimentally infected with porcine reproductive and respiratory syndrome virus (PRRSV). J Comp Pathol. 2003, 129:205-12.
-
Costers S, Vanhee M, Van Breedam W, Van Doorsselaere J, Geldhof M, Nauwynck HJ. GP4-specific neutralizing antibodies might be a driving force in PRRSV evolution. Virus Res. 2010, 154:104-13.
-
Costers S, Lefebvre DJ, Goddeeris B, Delputte PL, Nauwynck HJ. Functional impairment of PRRSV-specific peripheral CD3+CD8high cells. Vet Res. 2009, 40:46.
-
Darwich L, Díaz I, Mateu E. Certainties, doubts and hypotheses in porcine reproductive and respiratory syndrome virus immunobiology. Virus Res. 2010, 154:123-32.
-
Darwich L, Gimeno M, Sibila M, Diaz I, de la Torre E, Dotti S, Kuzemtseva L, Martin M, Pujols J, Mateu E. Genetic and immunobiological diversities of porcine reproductive and respiratory syndrome genotype I strains. Vet Microbiol. 2011, 150:49-62.
-
de Lima M, Pattnaik AK, Flores EF, Osorio FA. Serologic marker candidates identified among B-cell linear epitopes of Nsp2 and structural proteins of a North American strain of porcine reproductive and respiratory syndrome virus. Virology. 2006, 30:410–421.
-
Díaz I, Darwich L, Pappaterra G, Pujols J, Mateu E. Immune responses of pigs after experimental infection with a European strain of Porcine reproductive and respiratory syndrome virus. J Gen Virol. 2005, 86:1943-51.
-
Díaz I, Darwich L, Pappaterra G, Pujols J, Mateu E. Different European-type vaccines against porcine reproductive and respiratory syndrome virus have different immunological properties and confer different protection to pigs. Virology. 2006, 351:249-59.
-
Díaz I, Pujols J, Ganges L, Gimeno M, Darwich L, Domingo M, Mateu E. In silico prediction and ex vivo evaluation of potential T-cell epitopes in glycoproteins 4 and 5 and nucleocapsid protein of genotype-I (European) of porcine reproductive and respiratory syndrome virus. Vaccine. 2009, 27:5603-11.
-
Díaz I, Gimeno M, Darwich L, Navarro N, Kuzemtseva L, López S, Galindo I, Segalés J, Martín M, Pujols J, Mateu E. Characterization of homologous and heterologous adaptive immune responses in porcine reproductive and respiratory syndrome virus infection. Vet Res. 2012, 19:43:30.
-
Dokland T. The structural biology of PRRSV. Virus Res. 2010, 154:86-97.
-
Dwivedi V, Manickam C, Binjawadagi B, Linhares D, Murtaugh MP, Renukaradhya GJ. Evaluation of immune responses to porcine reproductive and respiratory syndrome virus in pigs during early stage of infection under farm conditions. Virol J. 2012, 9:45.
-
EvansAB, Loyd H, Dunkelberger JR, van Tol S, Bolton MJ, Dorman KS, Dekkers JCM, Carpenter S. Antigenic and Biological Characterization of ORF2-6 Variants at Early Times Following PRRSV Infection. 2017, 16;9(5). pii: E113. doi: 10.3390/v9050113.
-
Faaberg KS, Hocker JD, Erdman MM, Harris DL, Nelson EA, Torremorell M, Plagemann PG. Neutralizing antibody responses of pigs infected with natural GP5 N-glycan mutants of porcine reproductive and respiratory syndrome virus. Viral Immunol. 2006, 19:294-304.
-
Fan B, Liu X, Bai J, Zhang T, Zhang Q, Jiang P. Influence of the amino acid residues at 70 in M protein of porcine reproductive and respiratory syndrome virus on viral neutralization susceptibility to the serum antibody. Virol J. 2016, 22:51. doi: 10.1186/s12985-016-0505-7.
-
Fang L, Jiang Y, Xiao S, Niu C, Zhang H, Chen H. Enhanced immunogenicity of the modified GP5 of porcine reproductive and respiratory syndrome virus. Virus Genes. 2006, 32:5–11.
-
Gimeno M, Darwich L, Diaz I, de la Torre E, Pujols J, Martín M, Inumaru S, Cano E, Domingo M, Montoya M, Mateu E. Cytokine profiles and phenotype regulation of antigen presenting cells by genotype-I porcine reproductive and respiratory syndrome virus isolates. Vet Res. 2011, 18:42:9.
-
Gómez-Laguna J, Salguero FJ, Pallarés FJ, Carrasco L. Immunopathogenesis of porcine reproductive and respiratory syndrome in the respiratory tract of pigs. Vet J. 2013, 195:148-55.
-
Gómez-Laguna J, Salguero FJ, Barranco I, Pallarés FJ, Rodríguez-Gómez IM, Bernabé A, Carrasco L. Cytokine expression by macrophages in the lung of pigs infected with the porcine reproductive and respiratory syndrome virus. J Comp Pathol. 2010, 142:51-60.
-
GuW, Guo L, Yu H, Niu J, Huang M, Luo X, Li R, Tian Z, Feng L, Wang Y. Involvement of CD16 in antibody-dependent enhancement of porcine reproductive and respiratory syndrome virus infection. J Gen Virol. 2015, 96:1712-22.
-
Han M, Kim CY, Rowland RR, Fang Y, Kim D, Yoo D. Biogenesis of non-structural protein 1 (nsp1) and nsp1-mediated type I interferon modulation in arteriviruses. Virology. 2014, 458-459:136-50.
-
He Q, Li Y, Zhou L, Ge X, Guo X, Yang H. Both Nsp1β and Nsp11 are responsible for differential TNF-α production induced by porcine reproductive and respiratory syndrome virus strains with different pathogenicity in vitro. Virus Res. 2015, 201:32-40.
-
Huang C, Zhang Q, Guo XK, Yu ZB, Xu AT, Tang J, Feng WH. Porcine reproductive and respiratory syndrome virus nonstructural protein 4 antagonizes beta interferon expression by targeting the NF-κB essential modulator. J Virol. 2014, 88:10934-45.
-
Ke H, Yoo D. The viral innate immune antagonism and an alternative vaccine design for PRRS virus. Vet Microbiol. 2017, 209:75-89.
-
Kim WI, Lee DS, Johnson W, Roof M, Cha SH, Yoon KJ. Effect of genotypic and biotypic differences among PRRS viruses on the serologic assessment of pigs for virus infection. Vet Microbiol. 2007, 123:1-14.
-
Kimman TG, Cornelissen LA, Moormann RJ, Rebel JM, Stockhofe-Zurwieden N. Challenges for porcine reproductive and respiratory syndrome virus (PRRSV) vaccinology. Vaccine. 2009, 27:3704-18.
-
Kuzemtseva L, de la Torre E, Martín G, Soldevila F, Ait-Ali T, Mateu E, Darwich L. Regulation of toll-like receptors 3, 7 and 9 in porcine alveolar macrophages by different genotype 1 strains of porcine reproductive and respiratory syndrome virus. Vet Immunol Immunopathol. 2014, 158:189-98.
-
Lager KM, Mengeling WL, Brockmeier SL. Homologous challenge of porcine reproductive and respiratory syndrome virus immunity in pregnant swine. Vet Microbiol. 1997, 58:113-25.
-
Lamontagne L, Page C, Larochelle R, Longtin D, Magar R. Polyclonal activation of B cells occurs in lymphoid organs from porcine reproductive and respiratory syndrome virus (PRRSV)-infected pigs. Vet Immunol Immunopathol. 2001, 82:165-82.
-
Lamontagne L, Page C, Larochelle R, Magar R. Porcine reproductive and respiratory syndrome virus persistence in blood, spleen, lymph nodes, and tonsils of experimentally infected pigs depends on the level of CD8high T cells. Viral Immunol. 2003, 16:395-406.
-
Lee SM, Schommer SK, Kleiboeker SB. Porcine reproductive and respiratory syndrome virus field isolates differ in in vitro interferon phenotypes. Vet Immunol Immunopathol. 2004, 102:217-31.
-
Li Y, Zhu L, Lawson SR, Fang Y. Targeted mutations in a highly conserved motif of the nsp1β protein impair the interferon antagonizing activity of porcine reproductive and respiratory syndrome virus. J Gen Virol. 2013, 94:1972-83.
-
Loemba HD1, Mounir S, Mardassi H, Archambault D, Dea S. Kinetics of humoral immune response to the major structural proteins of the porcine reproductive and respiratory syndrome virus. Arch Virol. 1996, 141:751-61.
-
Lohse L, Nielsen J, Eriksen L. Temporary CD8+ T-cell depletion in pigs does not exacerbate infection with porcine reproductive and respiratory syndrome virus (PRRSV). Viral Immunol. 2004, 17:594-603.
-
Lopez OJ, Osorio FA. Role of neutralizing antibodies in PRRSV protective immunity. Vet Immunol Immunopathol. 2004, 102:155-63.
-
Loving CL, Brockmeier SL, Sacco RE. Differential type I interferon activation and susceptibility of dendritic cell populations to porcine arterivirus. Immunology. 2007, 120:217-29.
-
Lowe JE, Husmann R, Firkins LD, Zuckermann FA, Goldberg TL. Correlation of cell-mediated immunity against porcine reproductive and respiratory syndrome virus with protection against reproductive failure in sows during outbreaks of porcine reproductive and respiratory syndrome in commercial herds. J Am Vet Med Assoc. 2005, 226:1707-11.
-
Lunney JK, Benfield DA, Rowland RR. Porcine reproductive and respiratory syndrome virus: an update on an emerging and re-emerging viral disease of swine. Virus Res. 2010, 154:1-6.
-
Lunney JK, Fang Y, Ladinig A, Chen N, Li Y, Rowland B, Renukaradhya GJ. Porcine reproductive and respiratory syndrome virus (PRRSV): Pathogenesis and Interaction with the Immune System. Annu Rev Anim Biosci. 2016, 4:129-54
-
Magar R, Larochelle R, Nelson EA, Charreyre C. Differential reactivity of a monoclonal antibody directed to the membrane protein of porcine reproductive and respiratory syndrome virus. Can J Vet Res. 1997, 61:69-71.
-
Martelli P, Gozio S, Ferrari L, Rosina S, De Angelis E, Quintavalla C, Bottarelli E, Borghetti P. Efficacy of a modified live porcine reproductive and respiratory syndrome virus (PRRSV) vaccine in pigs naturally exposed to a heterologous European (Italian cluster) field strain: Clinical protection and cell-mediated immunity. Vaccine. 2009, 27:3788-99.
-
Martínez-Lobo FJ, Díez-Fuertes F, Simarro I, Castro JM, Prieto C. Porcine Reproductive and Respiratory Syndrome Virus isolates differ in their susceptibility to neutralization. Vaccine. 2011, 29:6928-40.
-
Mateu E, Diaz I. The challenge of PRRS immunology. Vet J. 2008, 177:345-51.
-
Meier WA, Galeota J, Osorio FA, Husmann RJ, Schnitzlein WM, Zuckermann FA. Gradual development of the interferon-gamma response of swine to porcine reproductive and respiratory syndrome virus infection or vaccination. Virology. 2003, 309:18-31.
-
Mengeling WL, Lager KM, Vorwald AC, Koehler KJ. Strain specificity of the immune response of pigs following vaccination with various strains of porcine reproductive and respiratory syndrome virus. Vet Microbiol. 2003, 93:13-24.
-
Meulenberg JJ, van Nieuwstadt AP, van Essen-Zandbergen A, Bos-de Ruijter JN, Langeveld JP, Meloen RH. Localization and fine mapping of antigenic sites on the nucleocapsid protein N of porcine reproductive and respiratory syndrome virus with monoclonal antibodies. Virology. 1998, 252:106-14.
-
Murtaugh MP, Xiao Z, Zuckermann F. Immunological responses of swine to porcine reproductive and respiratory syndrome virus infection. Viral Immunol. 2002, 15:533-47.
-
Murtaugh MP, Genzow M. Immunological solutions for treatment and prevention of porcine reproductive and respiratory syndrome (PRRS). Vaccine. 2011, 29:8192-204.
-
Murtaugh MP, Stadejek T, Abrahante JE, Lam TT, Leung FC. The ever-expanding diversity of porcine reproductive and respiratory syndrome virus. Virus Res. 2010, 154:18-30.
-
Nauwynck HJ, Van Gorp H, Vanhee M, Karniychuk U, Geldhof M, Cao A, Verbeeck M, Van Breedam W. Micro-dissecting the pathogenesis and immune response of PRRSV infection paves the way for more efficient PRRSV vaccines. Transbound Emerg Dis. 2012, 1:50-4.
-
Nelson EA, Christopher-Hennings J, Benfield DA. Serum immune responses to the proteins of porcine reproductive and respiratory syndrome (PRRS) virus. J Vet Diagn Invest. 1994, 6:410-5.
-
Oleksiewicz MB, Bøtner A, Toft P, Normann P, Storgaard T. Epitope mapping porcine reproductive and respiratory syndrome virus by phage display: the nsp2 fragment of the replicase polyprotein contains a cluster of B-cell epitopes. J Virol. 2001, 75:3277-90.
-
Ostrowski M, Galeota JA, Jar AM, Platt KB, Osorio FA, Lopez OJ. Identification of neutralizing and nonneutralizing epitopes in the porcine reproductive and respiratory syndrome virus GP5 ectodomain. J Virol. 2002, 76:4241-50.
-
Rose N, Renson P, Andraud M, Paboeuf F, Le Potier MF, Bourry O. Porcine reproductive and respiratory syndrome virus (PRRSv) modified-live vaccine reduces virus transmission in experimental conditions. Vaccine. 2015, 33:2493-9.
-
Pileri E, Gibert E, Soldevila F, García-Saenz A, Pujols J, Diaz I, Darwich L, Casal J, Martín M, Mateu E. Vaccination with a genotype 1 modified live vaccine against porcine reproductive and respiratory syndrome virus significantly reduces viremia, viral shedding and transmission of the virus in a quasi-natural experimental model. Vet Microbiol. 2015, 175:7-16.
-
Pirzadeh B, Dea S. Monoclonal antibodies to the ORF5 product of porcine reproductive and respiratory syndrome virus define linear neutralizing determinants. J Gen Virol. 1997, 78: 1867–73.
-
Prieto C, Alvarez E, Martínez-Lobo FJ, Simarro I, Castro JM. Similarity of European porcine reproductive and respiratory syndrome virus strains to vaccine strain is not necessarily predictive of the degree of protective immunity conferred. Vet J. 2008, 175:356-63.
-
Rascón-Castelo E, Burgara-Estrella A, Mateu E, Hernández J. Immunological features of the non-structural proteins of porcine reproductive and respiratory syndrome virus. Viruses. 2015, 7:873-86.
-
Reiner G. Genetic resistance – an alternative for controlling PRRS? Porcine Health Manag. 2016, 2:27.
-
Ren JQ, Sun WC, Lu HJ1, Wen SB, Jing J, Yan FL, Liu H, Liu CX, Xiao PP, Chen X, Du SW, Du R, Jin NY. Construction and immunogenicity of a DNA vaccine coexpressing GP3 and GP5 of genotype-I porcine reproductive and respiratory syndrome virus. BMC Vet Res. 2014, 10:128.
-
Ren J, Lu H, Wen S, Sun W, Yan F, Chen X, Jing J, Liu H, Liu C, Xue F, Xiao P, Xin S, Jin N. Enhanced immune responses in pigs by DNA vaccine coexpressing GP3 and GP5 of European type porcine reproductive and respiratory syndrome virus. J Virol Methods. 2014, 206:27-37.
-
Roca M, Gimeno M, Bruguera S, Segalés J, Díaz I, Galindo-Cardiel IJ, Martínez E, Darwich L, Fang Y, Maldonado J, March R, Mateu E. Effects of challenge with a virulent genotype II strain of porcine reproductive and respiratory syndrome virus on piglets vaccinated with an attenuated genotype I strain vaccine. Vet J. 2012, 193:92-6.
-
Rodríguez-Gómez IM, Gómez-Laguna J, Carrasco L. Impact of PRRSV on activation and viability of antigen presenting cells. World J Virol. 201, 2:146-51.
-
Royaee AR, Husmann RJ, Dawson HD, Calzada-Nova G, Schnitzlein WM, Zuckermann FA, Lunney JK. Deciphering the involvement of innate immune factors in the development of the host response to PRRSV vaccination. Vet Immunol Immunopathol. 2004, 102:199-216.
-
Samsom JN, de Bruin TG, Voermans JJ, Meulenberg JJ, Pol JM, Bianchi AT. Changes of leukocyte phenotype and function in the broncho-alveolar lavage fluid of pigs infected with porcine reproductive and respiratory syndrome virus: a role for CD8(+) cells. J Gen Virol. 2000, 81:497-505.
-
Scortti M, Prieto C, Martínez-Lobo FJ, Simarro I, Castro JM. Effects of two commercial European modified-live vaccines against porcine reproductive and respiratory syndrome viruses in pregnant gilts. Vet J. 2006, 172:506-14.
-
Shimizu M, Yamada S, Kawashima K, Ohashi S, Shimizu S, Ogawa T. Changes of lymphocyte subpopulations in pigs infected with porcine reproductive and respiratory syndrome (PRRS) virus. Vet Immunol Immunopathol. 1996, 50:19-27.
-
Silva-Campa E, Mata-Haro V, Mateu E, Hernández J. Porcine reproductive and respiratory syndrome virus induces CD4+CD8+CD25+Foxp3+ regulatory T cells (Tregs). Virology. 2012, 430:73-80.
-
Sun Y, Han M, Kim C, Calvert JG, Yoo D. Interplay between interferon-mediated innate immunity and porcine reproductive and respiratory syndrome virus. Viruses. 2012, 4:424-46.
-
Takikawa N, Kobayashi S, Ide S, Yamane Y, Tanaka Y, Yamagishi H. Detection of antibodies against porcine reproductive and respiratory syndrome (PRRS) virus in swine sera by enzyme-linked immunosorbent assay. J Vet Med Sci. 1996, 56: 355–357.
-
van der Linden IF, Voermans JJ, van der Linde-Bril EM, Bianchi AT, Steverink PJ. Virological kinetics and immunological responses to a porcine reproductive and respiratory syndrome virus infection of pigs at different ages. Vaccine. 2003, 21:1952-7.
-
Vanhee M, Van Breedam W, Costers S, Geldhof M, Noppe Y, Nauwynck H. Characterization of antigenic regions in the porcine reproductive and respiratory syndrome virus by the use of peptide-specific serum antibodies. Vaccine. 2011, 29:4794-804.
-
Vanhee M, Costers S, Van Breedam W, Geldhof MF, Van Doorsselaere J, Nauwynck HJ. A variable region in GP4 of European-type porcine reproductive and respiratory syndrome virus induces neutralizing antibodies against homologous but not heterologous virus strains. Viral Immunol. 2010, 23:403-13.
-
Van Reeth K, Labarque G, Nauwynck H, Pensaert M. Differential production of proinflammatory cytokines in the pig lung during different respiratory virus infections: correlations with pathogenicity. Res Vet Sci. 1999, 67:47-52.
-
Vézina SA, Loemba H, Fournier M, Dea S, Archambault D. Antibody production and blastogenic response in pigs experimentally infected with porcine reproductive and respiratory syndrome virus. Can J Vet Res. 1996, 60:94-9.
-
Walsh KP, Mills KH. Dendritic cells and other innate determinants of T helper cell polarisation. Trends Immunol. 2013, 34:521-30.
-
Wang G, Song T, Yu Y, Liu Y, Shi W, Wang S, Rong F, Dong J, Liu H, Cai X, Zhou EM. Immune responses in piglets infected with highly pathogenic porcine reproductive and respiratory syndrome virus. Vet Immunol Immunopathol. 2011, 142:170-8.
-
Wang R, Nan Y, Yu Y, Yang Z, Zhang YJ. Variable interference with interferon signal transduction by different strains of porcine reproductive and respiratory syndrome virus. Vet Microbiol. 2013, 166:493-503.
-
Weesendorp E, Morgan S, Stockhofe-Zurwieden N, Popma-De Graaf DJ, Graham SP, Rebel JM. Comparative analysis of immune responses following experimental infection of pigs with European porcine reproductive and respiratory syndrome virus strains of differing virulence. Vet Microbiol. 2013, 163:1-12.
-
Yang L, Frey ML, Yoon KJ, Zimmerman JJ, Platt KB. Categorization of North American porcine reproductive and respiratory syndrome viruses: epitopic profiles of the N, M, GP5 and GP3 proteins and susceptibility to neutralization. Arch Virol. 2000, 145: 1599–619.
-
Yoon IJ, Joo HS, Christianson WT, Kim HS, Collins JE, Morrison RB, Dial GD. An indirect fluorescent antibody test for the detection of antibody to swine infertility and respiratory syndrome virus in swine sera. J Vet Diagn Inv. 1992, 4:144–7.
-
Yoon IJ, Joo HS, Goyal SM, Molitor TW. A modified serum neutralization test for the detection of antibody to porcine reproductive and respiratory syndrome virus in swine sera. J Vet Diagn Invest. 1994, 6:289-92.
-
Yoon KJ, Zimmerman JJ, Swenson SL, McGinley MJ, Eernisse KA, Brevik A, Rhinehart LL, Frey ML, Hill HT, Platt KB. Characterization of the humoral immune response to porcine reproductive and respiratory syndrome (PRRS) virus infection. J Vet Diagn Invest. 1995, 7:305-12.
-
Yoon KJ, Wu LL, Zimmerman JJ, Hill HT, Platt KB. Antibody-dependent enhancement (ADE) of porcine reproductive and respiratory syndrome virus (PRRSV) infection in pigs. Viral Immunol. 1996, 9:51-63.
-
Yoon KJ, Wu LL, Zimmerman JJ, Platt KB. Field isolates of porcine reproductive and respiratory syndrome virus (PRRSV) vary in their susceptibility to antibody dependent enhancement (ADE) of infection. Vet Microbiol. 1997, 55:277-87.
-
Zimmerman JJ, Benfield DA, Dee SA, Murtaugh MP, Stadejek T, Stevenson GW, Torremorell M. Porcine reproductive and respiratory syndrome virus (porcine arterivirus). In: 10th ed. Diseases of swine, Ed. Wiley-Blackwell. 2012, 31:463-86.